SUBHEAD: Recent research is improving electric battery storage performance and efficency.
By Jan TenBruggencate on 27 September 2015 for Raising Islands -
(http://raisingislands.blogspot.com/2015/09/battery-technology-exploding-in-good-way.html)
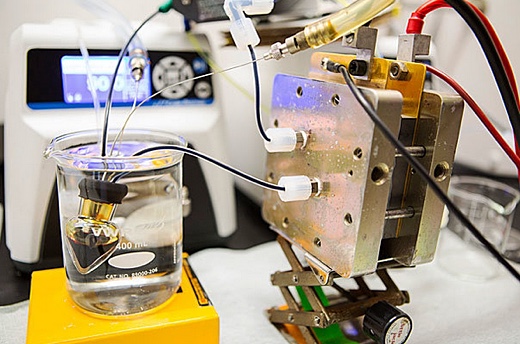
Image above: A cheap, common materials that “deliver the first high-performance, non-flammable, non-toxic, non-corrosive and low-cost chemicals for flow batteries. From (http://www.sciencedaily.com/releases/2015/09/150924151401.htm).
If the future of energy is the ability to cheaply and safely store intermittent renewables, then there’s lots of good news on the battery front.
Renewable plus storage means decarbonizing the grid. Public policy argues that’s something we should do effectively and as quickly as possible.
One of the problems is that batteries haven’t been up to the task. Some are too expensive, some are too toxic, some are too fragile, some lose too much energy charging and discharging, some die too soon and need to be replaced.
The list goes on.
But there’s amazing work being done globally on battery technology. We have reviewed some of that in an earlier series that starts here (http://raisingislands.blogspot.com/2013/08/hawaii-energy-storage-many-storage.html).
But since that 2013 series, there’s lots of new stuff. We’ll review a couple of innovations here, starting with flow batteries.
Video above: Flow battery explanation. From (https://youtu.be/4ob3_8QjmR0).
Researchers at Harvard have been looking for non-toxic alternatives to flow batteries using bromide electrolytes. Flow batteries have energy-rich electrolytes in external tanks, and the electrolytes are pumped through the battery, meaning battery capacity can be increased simply by increasing electrolyte storage.
“Harvard chemistry professor Roy Gordon said they found a formulation using cheap, common materials that “deliver the first high-performance, non-flammable, non-toxic, non-corrosive and low-cost chemicals for flow batteries. They reported their research in the Sept. 25 issue of Science (http://www.sciencemag.org/content/349/6255/1529).
Project chief investigator Michael Aziz said it would be a great way to store solar power: “"This is chemistry I'd be happy to put in my basement. See (http://www.sciencedaily.com/releases/2015/09/150924151401.htm).
The non-toxicity and cheap, abundant materials placed in water solution mean that it's safe -- it can't catch on fire -- and that's huge when you're storing large amounts of electrical energy anywhere near people."
Scientists at Ohio State have combined a solar cell and a battery in what they’re calling an aqueous solar flow battery. It’s still a ways from commercial production, but its inventors believe it has a lot of potential.
"This solar flow battery design can potentially be applied for grid-scale solar energy conversion and storage, as well as producing 'electrolyte fuels' that might be used to power future electric vehicles," said lead author Mingzhe Yu. They reported their findings in the Journal of the American Chemical Society (http://pubs.acs.org/doi/abs/10.1021/jacs.5b03626).
Stanford researchers have written about their new aluminum battery, which they say is fast-charging, inexpensive and lasts a long time. They believe it can replace alkaline and lithium-ion batteries. How fast a charge? Think about charging a cell phone in a minute, and a battery that can handle daily charging for decades.
The battery has an aluminum anode and graphite cathode in a liquid salt electrolyte. It still needs some work, but shows great potential, its inventors say.
And it’s not just for small electronics. “The grid needs a battery with a long cycle life that can rapidly store and release energy. See (http://www.sciencedaily.com/releases/2015/04/150406121031.htm). Our latest unpublished data suggest that an aluminum battery can be recharged tens of thousands of times,” said Stanford chemistry professor Hongjie Dai.
But this isn’t to say that all the research is on new battery technologies. There’s also still a lot of work underway on improving existing batteries. As an example, South Korean researchers are reporting on a new lithium-ion design that improves its performance while reducing the problem of overheating. See (http://www.sciencedaily.com/releases/2015/04/150406121031.htm).
And MIT researchers say they’ve developed a way to cut in half the cost of building lithium-ion batteries. That, and they work better, too. See (http://www.sciencedirect.com/science/article/pii/S0378775315010575).
Furthermore, there’s research underway in figuring out how to improve the amount of energy lost in charging and discharging a battery. Generally, you can lose 20 percent or more of the energy it takes to charge a battery when to draw that energy back out.
Researchers at Case Western Reserve University say they’ve adapted solar cells to dramatically increase that efficiency. They wired four perovskite solar cells in series and were able to charge a lithium-ion battery with 7.8 percent loss—the best performance seen to date, they say.
The Science Daily report on the work is here but it’s a little technical. See (http://www.sciencedaily.com/releases/2015/08/150827111639.htm) and (http://www.sciencedaily.com/releases/2015/08/150801082647.htm).
Perovskite solar cells are comparatively new on the solar scene. They can be manufactured inexpensively, and reportedly can convert into electricity a larger proportion of the sun’s light than other solar panels.
A lot of folks have wondered whether supercapacitors can be adapted to provide long-term energy storage. Supercapacitors are units that can store a lot of power, but they discharge almost instantaneously. Great for a sudden need for power—like when a motor starts up—but less useful as a continuing source of energy.
But there are a lot of applications for bursts of energy that are inefficiently met with standard batteries. Researchers at Department of Energy's Oak Ridge National Laboratory and Drexel University looked at new ways to use water materials—specifically old tires—in the manufacture of supercapacitors. See (http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500866/abstract;jsessionid=3161B665900A259D95A41E521A372F4F.f02t02).
The point here has not been to cover the universe of battery innovation, but to show that there’s a lot going on. Some of this stuff may not pan out, but a lot of it will, and it will change the energy landscape.
Some of these technologies may end up in our phones, in our cars, in our houses, out on our utility grids--and maybe even in places where we've never imagined a role for energy storage.
.
By Jan TenBruggencate on 27 September 2015 for Raising Islands -
(http://raisingislands.blogspot.com/2015/09/battery-technology-exploding-in-good-way.html)

Image above: A cheap, common materials that “deliver the first high-performance, non-flammable, non-toxic, non-corrosive and low-cost chemicals for flow batteries. From (http://www.sciencedaily.com/releases/2015/09/150924151401.htm).
If the future of energy is the ability to cheaply and safely store intermittent renewables, then there’s lots of good news on the battery front.
Renewable plus storage means decarbonizing the grid. Public policy argues that’s something we should do effectively and as quickly as possible.
One of the problems is that batteries haven’t been up to the task. Some are too expensive, some are too toxic, some are too fragile, some lose too much energy charging and discharging, some die too soon and need to be replaced.
The list goes on.
But there’s amazing work being done globally on battery technology. We have reviewed some of that in an earlier series that starts here (http://raisingislands.blogspot.com/2013/08/hawaii-energy-storage-many-storage.html).
But since that 2013 series, there’s lots of new stuff. We’ll review a couple of innovations here, starting with flow batteries.
Video above: Flow battery explanation. From (https://youtu.be/4ob3_8QjmR0).
Researchers at Harvard have been looking for non-toxic alternatives to flow batteries using bromide electrolytes. Flow batteries have energy-rich electrolytes in external tanks, and the electrolytes are pumped through the battery, meaning battery capacity can be increased simply by increasing electrolyte storage.
“Harvard chemistry professor Roy Gordon said they found a formulation using cheap, common materials that “deliver the first high-performance, non-flammable, non-toxic, non-corrosive and low-cost chemicals for flow batteries. They reported their research in the Sept. 25 issue of Science (http://www.sciencemag.org/content/349/6255/1529).
Project chief investigator Michael Aziz said it would be a great way to store solar power: “"This is chemistry I'd be happy to put in my basement. See (http://www.sciencedaily.com/releases/2015/09/150924151401.htm).
The non-toxicity and cheap, abundant materials placed in water solution mean that it's safe -- it can't catch on fire -- and that's huge when you're storing large amounts of electrical energy anywhere near people."
Scientists at Ohio State have combined a solar cell and a battery in what they’re calling an aqueous solar flow battery. It’s still a ways from commercial production, but its inventors believe it has a lot of potential.
"This solar flow battery design can potentially be applied for grid-scale solar energy conversion and storage, as well as producing 'electrolyte fuels' that might be used to power future electric vehicles," said lead author Mingzhe Yu. They reported their findings in the Journal of the American Chemical Society (http://pubs.acs.org/doi/abs/10.1021/jacs.5b03626).
Stanford researchers have written about their new aluminum battery, which they say is fast-charging, inexpensive and lasts a long time. They believe it can replace alkaline and lithium-ion batteries. How fast a charge? Think about charging a cell phone in a minute, and a battery that can handle daily charging for decades.
The battery has an aluminum anode and graphite cathode in a liquid salt electrolyte. It still needs some work, but shows great potential, its inventors say.
And it’s not just for small electronics. “The grid needs a battery with a long cycle life that can rapidly store and release energy. See (http://www.sciencedaily.com/releases/2015/04/150406121031.htm). Our latest unpublished data suggest that an aluminum battery can be recharged tens of thousands of times,” said Stanford chemistry professor Hongjie Dai.
But this isn’t to say that all the research is on new battery technologies. There’s also still a lot of work underway on improving existing batteries. As an example, South Korean researchers are reporting on a new lithium-ion design that improves its performance while reducing the problem of overheating. See (http://www.sciencedaily.com/releases/2015/04/150406121031.htm).
And MIT researchers say they’ve developed a way to cut in half the cost of building lithium-ion batteries. That, and they work better, too. See (http://www.sciencedirect.com/science/article/pii/S0378775315010575).
Furthermore, there’s research underway in figuring out how to improve the amount of energy lost in charging and discharging a battery. Generally, you can lose 20 percent or more of the energy it takes to charge a battery when to draw that energy back out.
Researchers at Case Western Reserve University say they’ve adapted solar cells to dramatically increase that efficiency. They wired four perovskite solar cells in series and were able to charge a lithium-ion battery with 7.8 percent loss—the best performance seen to date, they say.
The Science Daily report on the work is here but it’s a little technical. See (http://www.sciencedaily.com/releases/2015/08/150827111639.htm) and (http://www.sciencedaily.com/releases/2015/08/150801082647.htm).
Perovskite solar cells are comparatively new on the solar scene. They can be manufactured inexpensively, and reportedly can convert into electricity a larger proportion of the sun’s light than other solar panels.
A lot of folks have wondered whether supercapacitors can be adapted to provide long-term energy storage. Supercapacitors are units that can store a lot of power, but they discharge almost instantaneously. Great for a sudden need for power—like when a motor starts up—but less useful as a continuing source of energy.
But there are a lot of applications for bursts of energy that are inefficiently met with standard batteries. Researchers at Department of Energy's Oak Ridge National Laboratory and Drexel University looked at new ways to use water materials—specifically old tires—in the manufacture of supercapacitors. See (http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500866/abstract;jsessionid=3161B665900A259D95A41E521A372F4F.f02t02).
The point here has not been to cover the universe of battery innovation, but to show that there’s a lot going on. Some of this stuff may not pan out, but a lot of it will, and it will change the energy landscape.
Some of these technologies may end up in our phones, in our cars, in our houses, out on our utility grids--and maybe even in places where we've never imagined a role for energy storage.
.
No comments :
Post a Comment